
The carrier EVs exert crucial biological effects on recipient cells, impacting immunity, pre-metastatic niche preparation, angiogenesis, cancer cell stemness and horizontal oncogene transfer.
SIGNALING TRANSDUCTION FLATICON DRIVERS
This family are one of the major drivers of cancer and is involved in several of the most frequent malignancies such as non-small cell lung cancer, breast cancer, colorectal cancer and ovarian cancer. EGFR belongs to a family of four homologous tyrosine kinase receptors (TKRs). This mechanistic understanding will be key to unravelling the functional consequences of direct anti-EGFR targeted and indirect EGFR-impacting cancer therapies on the secretion of pro-tumoural EVs and on their effects on drug resistance and microenvironment subversion.ĮGFR and some of the cognate ligands extensively traffic in extracellular vesicles (EVs) from different biogenesis pathways. We also elaborate on outstanding questions relating to EGFR-driven EV biogenesis and available methods to explore them. Here, we highlight how the spatiotemporal rules that regulate EGFR intracellular function intersect with and influence different EV biogenesis pathways and discuss key regulatory features and interactions of this interplay. EGFR regulates its own inclusion in EVs through feedback loops during disease progression and in response to challenges such as hypoxia, epithelial-to-mesenchymal transition and drugs. The receptor is involved in the biogenesis of specific EV subpopulations, it signals as an active cargo, and it can influence the uptake of EVs by recipient cells. To hijack EVs, EGFR needs to play multiple signalling roles in the life cycle of EVs.
When loaded in extracellular vesicles (EVs), EGFR is one of the key proteins involved in the transfer of information between parental cancer and bystander cells in the tumour microenvironment. We focus on the role of the protein complexes involved in EV genesis, and provide a comprehensive perspective of the contribution of these complexes to intracellular vesicle sorting of developmental signals for their extracellular secretion, reception and transduction.Įpidermal growth factor receptor (EGFR) takes centre stage in carcinogenesis throughout its entire cellular trafficking odyssey. In the present review, we discuss the most recent advances with regard to EV involvement in developmental signalling at a distance. Preparation of these ligands in EVs involves intracellular vesicle sorting in an endocytosis-dependent recycling process before secretion.
SIGNALING TRANSDUCTION FLATICON FREE
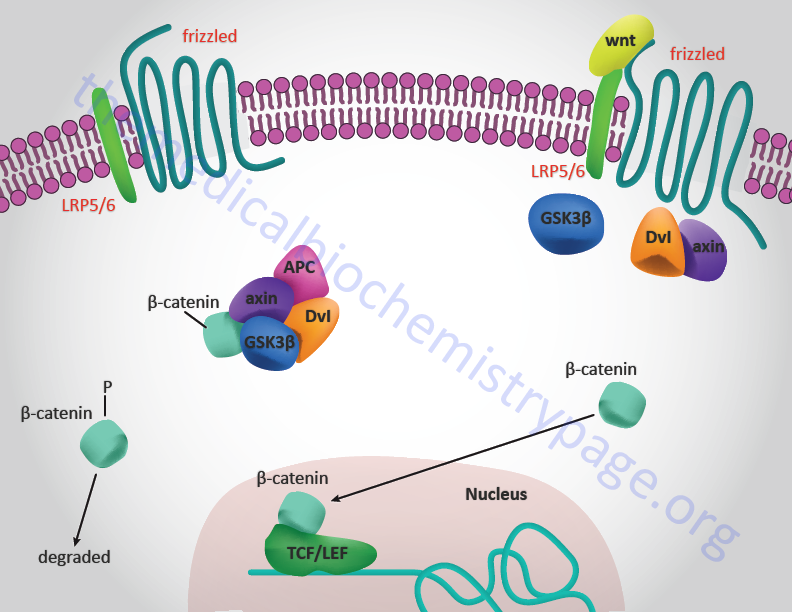
These signalling molecules undergo crucial post-translational lipid modifications, which anchor them to membranes and impede their free release into the extracellular space. EVs have recently been found to allow the transport of two major developmental signalling pathways: Hedgehog and Wnt. Extracellular vesicles (EVs) are membrane-based structures thought to facilitate the long-distance movement of signalling molecules. This communication can occur between adjacent and distant cells. Signalling from cell-to-cell is fundamental for determining differentiation and patterning.


 0 kommentar(er)
0 kommentar(er)
